
| New Technology Update | August 28, 2015 |
In This Edition
- Special Tech Issue
- Case Report: Konan EvokeDx
- Nutraceutical Review: Macuhealth
- Technology Review: OCULUS Keratograph® 5M
- Product Review: Avenova™
- Give Us Your Feedback
New Technology Update
The Gazette
Archives
Contact Vision Source® at 888-558-2020 or [email protected]
Special Tech Issue
Read What's New and Impressive in This Latest New Technology Update
It is amazing the number of new technologies that we are able to see, as we travel to the various meetings across the country. Most of these companies with new technologies are eager to offer these products and technologies to Vision Source® members. As a service to members, we will provide a periodic update to members on the offerings from existing and new vendors with new technologies.
These products and services will be evaluated in a clinical setting, and we will try and point out the applications and advantages of these technologies so that you can gauge for yourself whether it is the right fit for your practice.
 |
 |
John A. McCall, OD |
Carl Spear, OD, MBA, FAAO |
Case Report: Konan EvokeDx
Konan EvokeDx Helps to Clear Referral Questions

By Carl Spear, OD, MBA, FAAO
Special thanks to Jill Anderson, OD, and Robert Constantine, both from Sight & Sun Eyeworks, a Vision Source® member practice in Pensacola, Florida
A 51-year-old female patient presented to the clinic with a decrease in distance visual acuity. She’s been a patient since 2001, with a history of high astigmatism. Her usual BCVA of 20/60 OD and 20/50 OS had decreased to 20/150 BCVA. The patient was concerned, but neither DFE nor macular OCT showed any pathology. A 24-2 visual field showed no pattern deviation, only a general reduction consistent with decreased acuity. Patient has also reported a recent test indicating reduced nerve conduction in the lower extremities.
An MRI was ordered, which also showed no apparent pathology.

This was an excellent opportunity to put our newly installed Konan EvokeDx VEP/ERG to good use. The visual evoked potential goes beyond the static pictures and imaging usually seen in the clinical setting and offers a dynamic picture of the efficiency of the visual pathways. The VEP can help to identify visual problems that are pathway-based and give objective information on the functioning of the pathways. When compared to the ERG data, the VEP data can help direct clinical decision-making and help determine the possible cause of vision loss.
The trained technician was able to place the electrodes, following the instructions of the Konan trainer. The high-contrast, transient VEP was performed, as was the transient pattern ERG. The VEP showed normal N75 and P100 readings, indicating intact and efficient cortical pathways. The PERG showed reduced frequency response in both eyes, indicating retinal pathology as the cause of the decreased acuity. The Konan support team was able to log in in remotely to the device and confirm our results and conclusions.

This information allowed us to refer the patient to a retina specialist and provide him with a tremendous amount of clinical information. We are waiting on results of that visit, but we were able to conclusively rule out multiple sclerosis and make the appropriate referral in the best interest of the patient.
The Konan EvokeDx VEP/ERG has quickly become a valuable part of our diagnostic protocols. The support we receive from Konan has made the introduction of the device to the team members seamless. The multiple uses in treatment and management of a wide variety of diseases.
Nutraceutical Review: MacuHealth
MacuHealth Incorporates Findings Beyond AREDS2
By John McCall, OD, and Carl Spear, OD, MBA, FAAO

The Age Related Eye Disease Study 2 (AREDS2) demonstrated that supplementing the macula’s naturally occurring carotenoids is of visual benefit to patients with non-advanced age-related macular degeneration (AMD).
Since that important publication, however, further research has shown that a formulation containing all three of the macula’s carotenoids (lutein [L], zeaxanthin [Z] and meso-zeaxanthin [MZ]) offers many advantages over AREDS2-like formulations that contain only two of these carotenoids (L & Z).

First, head-to-head trials have shown that MacuHealth with LMZ3 (which contains all three carotenoids in a MZ:L:Z (mg) ratio of 10:10:2) results in greater augmentation of macular pigment (MP) and better enhancement of vision among subject with AMD when compared with a formulation lacking the centrally dominant MZ and that serum concentrations of MZ are the principal determinants of increases in MP following supplementation. These findings clearly have important implications for eye care professionals interested in optimizing the quality of life in their patients afflicted with AMD.
Further, MacuHealth remains the only formulation proven, in the context of a randomized, placebo-controlled trial, to improve vision in healthy subjects free of macular disease and to be superior to alternative formulations, with obvious important implications for consumers wishing to maximize their visual performance.

In summary, AREDS2 has shown that supplementation with the macula’s naturally occurring carotenoids is important for patients with AMD. Subsequent and emerging research has demonstrated that eye care professionals who want to augment their patients’ MP and enhance the visual performance and experience of their patients should use a formulation containing all three of the macular carotenoids in a MZ:L:Z (mg) ratio of 10:10:2 (MacuHealth). That’s true whether those patients suffer from AMD or are free of disease. We recommend MacuHealth for all of our patients who are at risk and who have early stages of AMD.
Technology Review: OCULUS Keratograph® 5M
The OCULUS Keratograph® 5M is Ideal for Diagnosing Dry Eye

Technology also helps with patient education and marketing
By Carl Spear, OD, MBA, FAAO
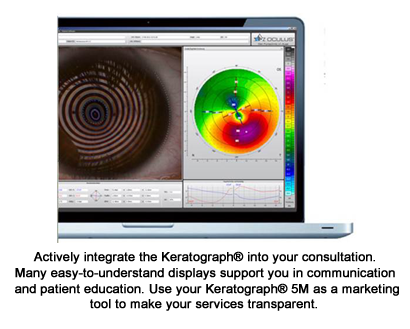
The OCULUS Keratograph® 5M is advanced technology that helps eye care professionals in accurately diagnosing dry eye syndrome and documenting the findings. If treatment is necessary, the Keratograph 5M can compare the before and after treatment results to evaluate the success of the treatment.
Here are the highlights of the Keratograph 5M technology.
JENVIS Dry Eye Report
The unique OCULUS JENVIS Dry Eye Report is a one-page summary report that shows the results of all dry eye examinations in a concise and meaningful format. It displays the data gathered from the Keratograph 5M dry eye exams, as well as the results of other examinations such as osmolarity, blink rate and OSDI dry eye questionnaire.
Meiboscan
Meibomian gland dysfunction (MGD) is the most common cause of dry eye disease. The Keratograph 5M can easily perform meibography of the upper and lower eyelids to show the morphological changes in glandular tissue that accompany the MGD.
Tear film scan: noninvasive tear film breakup time and tear film meniscus height measurements
The tear film breakup time is measured noninvasively and is displayed with an intuitive color map. The tear film meniscus height is measured precisely using the integrated measuring tool. The quality of the lipid layer and the tear film dynamics can be evaluated easily with various imaging and documentation modes. These features simplify the dry eye screening and provide digital documentation for progression control and to show the success of dry eye therapies.

R-scan: evaluating the redness of the eye
This instrument can document and classify the conjunctival and bulbar redness automatically.
High-resolution external imaging (color photos and video)
The built-in camera can take high-resolution photos and videos and is great for external photography and professional digital documentation. This robust system comes with a magnification changer. The light source can be changed depending on application. For example, white light source is used for color images; infrared is used for high-contrast, black and white images; and the blue light is used for fluorescein imaging. The external photography feature is reimbursable.
Topography and keratometry
The Keratograph 5M is a reliable corneal topographer with a real built-in keratometer for measuring the real Ks. It uses the placido technology and measures 22,000 points. It generates the axial and tangential curvature maps. The standard package comes with keratoconus detection software and contact lens fitting software. It even interfaces with third-party software such as for Paragon and WAVE contact lenses for specialty ortho-k lens fitting.
Patient education and marketing capabilities
Dry eye screening has been made easier with the advancements in technology. With the Keratograph 5M, it is easy to show the results to the patients and explain that dry eye syndrome is a progressive disease. Keratograph 5M images and numeric values can be the base for patient consultation and for suggesting a suitable course of treatment plan tailored to each patient’s unique case. Proper education fosters patient compliance and can support the appropriate recommendation for follow-up, billable visits.
Conclusion
Keratograph 5M is a unique diagnostic device that can help with discovering dry eye, categorizing the type of dry eye, summarizing all the findings in one report (including tests that are performed separately), explaining the condition to the patient, finding the best course of treatment and evaluating the post-treatment results.
Dry eye treatment starts with patient education, and the Keratograph 5M, with all its features, is the best educational tool in this market segment.
Product Review: Avenova™
Rx-only Avenova Hits the Mark for Long-term, Continuous, Daily Lid Hygiene


By John McCall, OD, and Carl Spear, OD, MBA, FAAO
If your patients complain of eyelid irritation, gritty sensation, crusting of lids, dryness, redness or fluctuations in vision, consider prescribing Avenova™. This revolutionary product is ideal as part of any lid hygiene regimen for MGD/dry eye, blepharitis (including Demodex), contact lens wear, ocular surgery and procedures and following makeup removal. It has 1,000 times lower toxicity when compared to Betadine, kills microorganisms in seconds and provides noticeable results within two weeks.

Avenova is the only nondetergent-based, prescription eyelid and lash hygiene product containing pure hypochlorous acid (HOCl), which NovaBay calls Neutrox™. The manufacturing process for Neutrox utilizes a patented, state-of-the-art technology for the production of pure HOCl. It is manufactured without impurities that can cause irritation. This product is stable for three years in amber glass.
All other HOCl products are impure and are either manufactured by Dakin’s process or by electrolysis, both of which produce significant amounts of sodium hypochlorite (NaOCl), commonly known as household bleach. A NaOCl product introduced during World War I was called Dakin’s solution.
Avenova does not contain surfactants, which are key ingredients in many lid scrub products and baby shampoo. Surfactants can dry the skin by stripping oils and can also cause a dermatitis. This unique product is nontoxic and nonsensitizing to the skin.
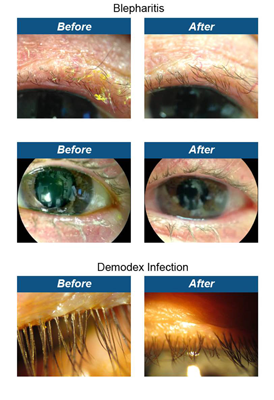
Avenova with Neutrox was designed for long-term, continuous, daily lid hygiene. As demonstrated by in vitro testing, a head-to-head study showed that Neutrox is less cytotoxic than another marketed product. Zone of Inhibition Tests revealed that Neutrox is more potent as an antimicrobial than NaOCl against S. aureus ATCC 29213. Currently, an estimated 40,000 patients can attest to the safety and efficacy of Avenova.
This product is available by prescription only. Products with prescription designation generally require a greater degree of data for U.S. Food and Drug Administration clearance. Performance data for Avenova is clearly stated in the product's package insert, and considerable additional information is available for your review.
In vitro testing shows that Avenova in solution is not only effective against a wide variety of bacteria but also neutralizes the toxins produced by bacteria, known to cause ocular irritation. It potentially reduces the bacterial load on the eyelids and has shown broad spectrum antimicrobial activity in vitro against several species including Acinetobacter, Bacillus oleronius, C. albicans, E. coli, P. aeruginosa, Methicillin-resistant S. aureus, S. epidermidis and Vancomycin-resistant Enterococcus. Avenova in solution rapidly kills >99.99% of each of these bacterial species in vitro within 60 seconds.
We have been amazed at the clinical impact of Avenova. Patient results and response has been incredible. This is a must-try for anyone in clinical practice.
Your Feedback Counts

Please take a moment to answer this two-question survey—even if you've done so before. It provides us the feedback to improve The Gazette.
©Vision Source L.P. 2015. All Rights Reserved.



